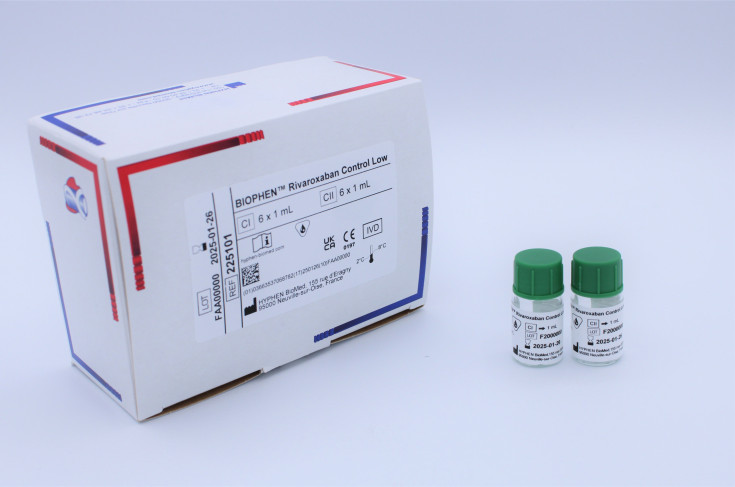
BIOPHEN™ Rivaroxaban Control Low
BIOPHEN™ Rivaroxaban Control Low
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
BIOPHEN™ Rivaroxaban Control Low (225101) is a Rivaroxaban quality control plasma set, low range. Manufactured by HYPHEN BioMed.
Product Overview:
BIOPHEN™ Rivaroxaban Control Low is intended for the quality control of quantitative, automated chromogenic assays that measure Rivaroxaban concentrations in human plasma. This kit is used for the quality control of low-range anti-Factor Xa (Anti-Xa) activity assays, including the BIOPHEN™ DiXaI (221030) and BIOPHEN™ Heparin LRT (221011/221013/221015) kits. In vitro diagnostic kit for professional use only.
Clinical Significance and Principle:
Rivaroxaban is an oral, direct inhibitor of Factor Xa. While routine therapeutic monitoring is generally not required, the quantification of its plasma concentration can be useful in specific clinical scenarios such as suspected overdosage, assessment of bleeding risk, or prior to emergency surgical procedures. This control plasma kit is used to monitor the accuracy and precision of the analytical method. It consists of lyophilized human plasmas containing known concentrations of Rivaroxaban, which are run alongside patient samples to verify the integrity of the test system.
Product Composition and Specifications:
The control kit is provided as lyophilized human plasma containing stabilizers. The plasma has been screened and found negative for HBsAg, anti-HIV-1, anti-HIV-2, and anti-HCV antibodies. The kit includes two controls for the low range:
- CI: Lyophilized human plasma containing approximately 25 ng/mL of Rivaroxaban.
- CII: Lyophilized human plasma containing approximately 80 ng/mL of Rivaroxaban.
Note: The exact concentration of each control is lot-specific. Users must refer to the values provided on the flyer included with the assay kit in use. The BIOPHEN™ Rivaroxaban Control Plasma (224501) is also available.
Traceability and Performance Characteristics:
For accurate results, the controls are traceable to the European Pharmacopoeia (Ph. Eur.) Certified Reference Standard for Rivaroxaban. A certificate of traceability is available upon request.
- Lot-to-Lot Variability: The coefficient of variation (%CV) between manufacturing lots is maintained at ≤ 10%.
- Measurement Uncertainty: The uncertainty for each control level is:
- CI: ± 2.4 ng/mL
- CII: ± 4.2 ng/mL
Storage and Stability:
- Unopened Vials: Store at 2-8°C. The product is stable until the expiration date printed on the label.
- Reconstituted Controls: When stored in a closed vial to prevent contamination and evaporation, the controls are stable for:
- 7 days at 2-8°C.
- 60 days when frozen at -20°C or below.
- Important: Reconstituted material should be thawed only once. Thaw rapidly at 37°C and use immediately.
- For on-board stability on automated analyzers, please refer to the specific Application Guide.
Quality Control:
In accordance with Good Laboratory Practice (GLP), quality control samples should be included in each series of tests to validate method compliance and ensure between-series assay homogeneity. If the controls fall outside of the established acceptance range, the series of assays must be invalidated and the analyses repeated after checking all system parameters.
Disclaimer:
The information on this page is for informational purposes and serves as a general summary. It is imperative that all users read and strictly follow the Instructions for Use (IFU) document provided with the reagent or kit they are using. This online description must not be a substitute for the official product documentation.
-
Product RangeProduct Code225101Product NameBIOPHEN™ Rivaroxaban Control LowProduct CategoryProduct BrandProduct Analyte or ApplicationProduct IVD Registration StatusCE-Mark - Professional Use
UKCAProduct Size2 x 6 x 1 ml -